OrgoChem I
Alkynes
Addition, hydration, reduction, oxidative cleavage, acetylide alkylation
Summary
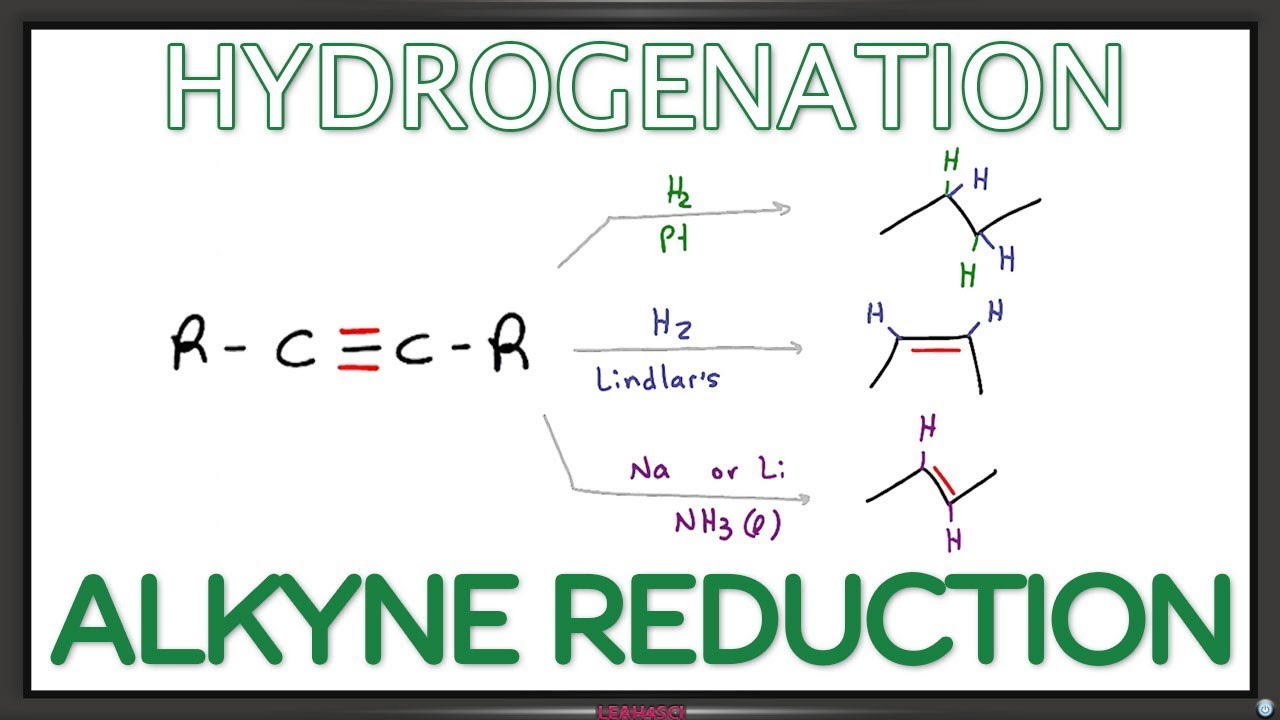
Alkynes add twice like alkenes. Hydration gives carbonyls via enol tautomerization. Lindlar → cis alkene; Na/NH₃ → trans. Terminal alkynes are acidic (pKa ~25).
What you need to know:
This topic covers alkynes. Focus on mastering the key concepts in the "Must know checklist" below. Follow the study steps systematically, and use practice problems to reinforce your understanding. The external reference provides comprehensive coverage of all concepts.
Video Tutorial
Must know checklist
0 / 5 complete
Common mistakes
Using NaOH to deprotonate terminal alkyne (need NaNH2 or BuLi; pKa ~25)
Confusing Lindlar (cis) vs Na/NH3 (trans) reduction products
Forgetting alkyne adds twice with HX or X2 (like alkene but 2×)
Internal alkyne + HX: regiochemistry ambiguous (both ends similar)