OrgoChem I
Resonance & Acid-Base
Drawing resonance forms, Brønsted-Lowry, pKa, conjugate pairs, equilibrium direction
Summary
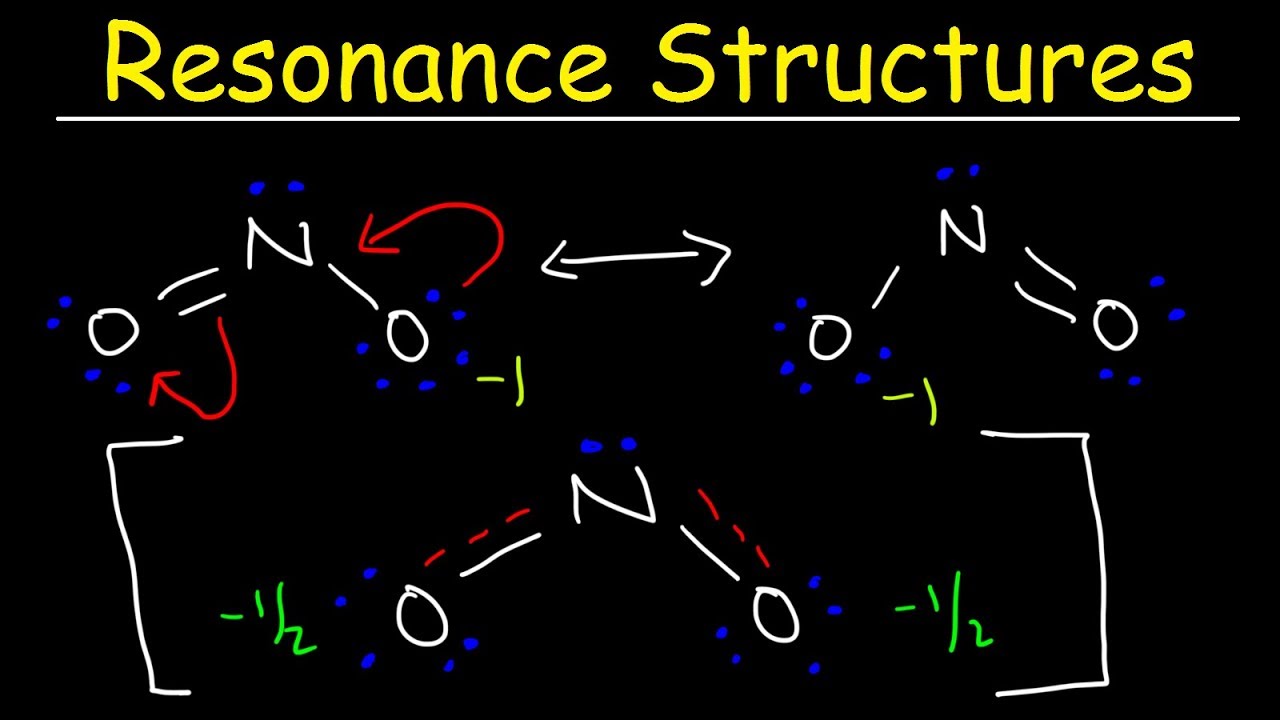
Master resonance (π bonds and lone pairs move; σ bonds stay) and acid-base (equilibrium favors weaker acid). Essential foundation for all of orgo.
What you need to know:
This topic covers resonance & acid-base. Focus on mastering the key concepts in the "Must know checklist" below. Follow the study steps systematically, and use practice problems to reinforce your understanding. The external reference provides comprehensive coverage of all concepts.
Video Tutorial
Must know checklist
0 / 5 complete
Common mistakes
Moving σ bonds in resonance (only π and lone pairs move)
Forgetting conjugate pairs are on the same side of equilibrium
Equilibrium favors stronger acid (wrong—favors weaker acid, higher pKa)
Not labeling all four: acid, base, conjugate acid, conjugate base